Interdisciplinary Initiatives Program Round 7 - 2014
Peter Kim, Biochemistry
James Swartz, Chemical Engineering, Bioengineering
Creating an AIDS vaccine remains a major unmet need -- worldwide, 35 million people (1 in 200) are infected with HIV. In spite of over 30 years of intense efforts, an HIV vaccine is still not in sight. Whole-killed vaccines, in which the virus is physically destroyed by chemicals, heat or radiation, do not lead to a relevant immune response. The natural immune system is difficult to activate because HIV infects precisely the cells that are required for the body to elicit a strong antibody response (CD4 positive T cells). In addition, the mutation rate of HIV is very high, so that variant strains of the virus can readily emerge that are resistant to a vaccine effective against only a single strain.
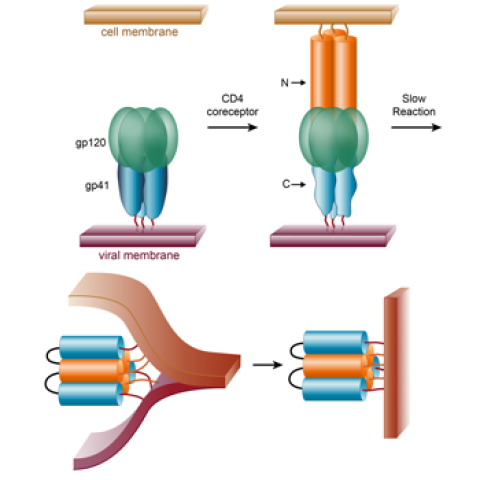
The proposed project aims to create an HIV vaccine in a novel manner, by targeting the process by which the virus infects cells. The HIV particle is surrounded by a membrane, similar to the membrane that surrounds a cell. In order to infect a cell, the viral membrane must fuse (i.e., become one) with the cell membrane. This membrane-fusion event is mediated by a specific protein on the surface of the virus (called gp41).
Earlier work by scientists has elucidated the overall features of this membrane-fusion event, including identification of a transient, but sensitive, intermediate state of the gp41 protein. This sensitive state is called the pre-hairpin intermediate. An FDA-approved drug, Fuzeon, works by binding to the HIV pre-hairpin intermediate. In addition, we have shown that a monoclonal antibody against the pre-hairpin intermediate can prevent infection of cells by diverse clinical isolates of HIV. Taken together, these results validate our approach to target the pre-hairpin intermediate as a vaccine target.
However, the monoclonal antibody referred to above was obtained in the laboratory. Numerous attempts, over many years, by us and others to create a vaccine candidate that allows animals to mount an effective antibody response against the HIV pre-hairpin intermediate have generally failed or led to weak responses. In the best case, using a hypersensitive virus (to increase our ability to detect a signal) we have been able to elicit an antibody response in animals that neutralizes HIV, but the neutralization activity is weak. These results were obtained with isolated peptides (short pieces of the gp41 protein) that fold to mimic the HIV pre-hairpin intermediate.
In the work proposed here, we will screen multiple variants of the previously evaluated pre-hairpin intermediates (the antigens) and present them to the immune system with multiple copies attached to the surface of specially engineered nanoparticles. Specific stimulators of the immune system will also be attached to elicit a more effective response. Although this approach is technically demanding, previous work has indicated feasibility for cancer vaccines. We now propose to synergistically combine the expertise of the Kim and Swartz labs to extend previous work and address this compelling global need.