Interdisciplinary Initiatives Program Round 7 - 2014
Carla Shatz, Biology
K Christopher Garcia, Molecular & Cellular Physiology
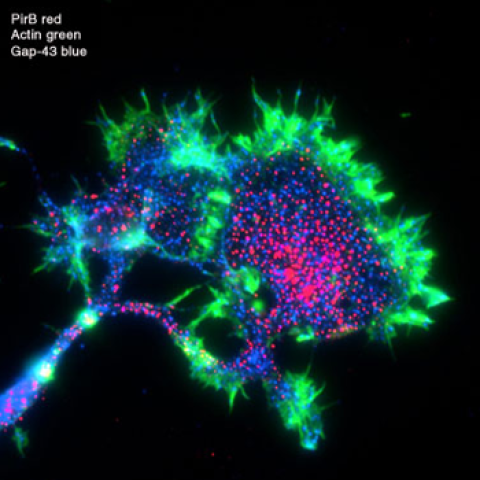
Alzheimer’s disease (AD) is the major cause of dementia in the elderly and is characterized by progressive memory loss and cognitive decline. A pathological hallmark of AD is accumulation of amyloid-beta (ABeta) deposits in the CNS. Soluble oligomeric species of ABeta are thought to be key mediators of synapse loss and cognitive dysfunction in AD. Recently, we discovered that ABeta oligomers bind to the receptor PirB (Paired immunoglobulin-like receptor B) and its human homologue LILRB2 in a specific, saturable manner (Kim et al, Science 2013). PirB-ABeta binding drives a downstream signaling pathway that leads to actin disassembly and synapse loss; the same pathway is hyperactivated in human AD brain. Notably, knockout of PirB prevents synaptic defects caused by ABeta, as well as memory loss in a transgenic mouse model of AD.
The major aim of this research proposal is to characterize and manipulate the ABeta-PirB interaction, with the ultimate purpose of developing novel inhibitors for therapeutic use. We propose a unique collaboration between the labs of K.C. Garcia and C.J. Shatz to accomplish this goal. These two labs bring together complimentary expertise in immunology and neurobiology, in structural biology and synaptic plasticity, and in vitro signaling and systems level in vivo studies of the CNS.
This unexpected discovery of a role for PirB in AD emerged from ongoing studies in the Shatz lab of activity-dependent synapse remodeling during normal developmental critical periods. During these periods, brain circuit connections are sculpted and pruned by experience. PirB was discovered in a search for molecules whose expression is regulated by neural activity and experience. It is expressed in neurons and plays key roles in regulating synaptic pruning and synaptic plasticity (Syken et al, 2006; Djurisic et al, 2013). The ABeta- PirB/LILRB2 receptor-ligand pair suggests a previously unknown mechanism contributing to AD pathology. In this proposed collaboration between the Shatz and Garcia Labs, we will characterize and study further this molecular interaction. The Garcia Lab is expert in structural biology and has extensive experience studying transmembrane receptors and their ligands. The Shatz Lab is experienced in functional and anatomical studies of synapse development both in vivo in mouse models and in vitro in neuronal culture systems. Three sets of experiments requiring the expertise of both labs are planned: 1) characterize the relationship between PirB binding to ABeta versus binding to normal endogenous ligands, 2) generate high affinity ABeta-PirB variants with the objective of obtaining structural information about the molecular basis for the interaction between ABeta and PirB, and 3) use the information gleaned from the first 2 sets of experiments to test the ability of high affinity ABeta variants to prevent hyperactivation of PirB signaling and consequent synapse disassembly even in the presence of soluble ABeta oligomers.
Together results from these experiments should not only broaden knowledge of the nature of the ABeta-PirB interaction, but should provide proof-of-principle in vivo for using optimized ABeta-PirB interfering peptides for therapeutic intervention in mouse models of AD. Given the high homology between PirB and LilrB2, the experiments would also pave the way for similar studies in human AD and possibly other neurological disorders in which synapse loss is a hallmark.