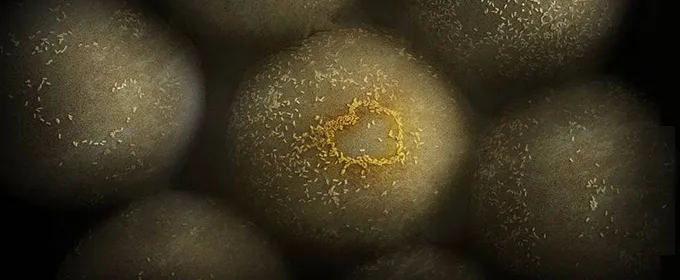
Photo by Yonatan Winetraub/Stanford School of Medicine: Minuscule gold nanoparticles glom onto and help identify tumor cells.
Stanford Engineering - May 11th, 2016 - by Ian Chipman
Anyone who's seen a loved one through a losing battle with cancer can attest to the range of emotions as treatments begin, offer hope and ultimately fail. "A lot of things go through your head. For me it was mostly outrage," says Adam de la Zerda, who watched a friend succumb to brain cancer. "How come this is the best that we can offer?"
A professor of structural biology and of electrical engineering at Stanford, de la Zerda is advancing a crucial front in the war on cancer. In a recent TEDx talk he outlined some of his lab's developments in medical imaging technology, and how the ground-breaking ability to see a clear picture of what's happening inside the body could lead to a dramatic transformation in how we detect and treat cancer.
We've been blindly fighting the war on cancer
De la Zerda notes two big changes in death rates from cancer since the 1930s. The first is that the number of patients dying from lung cancer has soared ("Thank you, cigarettes," he says). The second is that stomach cancer, which used to be the leading cause of cancer death in the U.S., has been all but eliminated. "Why is it that we are no longer struck by stomach cancer?" de la Zerda asks. "What was the huge medical technology breakthrough that came to our world that saved us from stomach cancer? Was it maybe a drug? Or a better diagnostic?"
It was neither of those things. Instead, advances in refrigeration meant we no longer ate spoiled meat. "So the best thing that happened to us so far in the medical arena of cancer research is the fact that the refrigerator was invented," de la Zerda says. "So we're not doing so well here."
While he doesn't minimize the progress of cancer research over the past 50 years, de la Zerda argues that until recently the effort was critically hamstrung. "A primary reason why we're not winning this war against cancer is because we're fighting blindly," he says. "This is where medical imaging comes in."
The most promising tool in the field of medical imaging is the PET/CT scan. For the first time, de la Zerda says, this technology allowed a non-invasive peek inside the body to see very clearly the location of a tumor or if the cancer has metastasized. "As miraculous as this may seem, unfortunately it's not that great."
To show up on a PET/CT scan, which works by tracking radioactive sugars injected into the patient, a tumor needs to have around 100 million cancer cells. "If that seems to you like a very large number, it is a very large number," de la Zerda says. In order to detect a tumor early enough to be able to do something meaningful about it, he suggests we would need to see tumors of only a thousand cells, or better yet, just a handful of cells in size.
"I remember looking at these numbers just being struck by the humongous difference between where we are today and where we need to be," de la Zerda says. So he went looking for a better way.
A new line of sight, courtesy of gold nanostructures
Along with collaborators at Stanford, de la Zerda has developed a promising technique that he hopes will provide a much clearer picture of, for instance, where a tumor ends and healthy tissue begins.
He says that brain surgeons face the nearly impossible task of deciding where to stop cutting when removing a tumor. Do you risk being too conservative, leaving unseen cancer cells behind, or do you cut out an extra inch of potentially healthy brain tissue just to be sure you got them all? With our current technology, de la Zerda says, it's essentially a guessing game. In fact, he says, the reason as much as 90% of brain cancer surgeries ultimately fail is that hidden leftover cancer cells grow and recur the tumor.
The novel approach developed by the team is to inject patients with billions of gold nanoparticles that are chemically programmed to seek out and stick to cancer cells while shining so that special cameras can see them. The researchers have successfully tested the technique in mice, identifying minuscule cancerous regions that the surgeon otherwise wouldn't have been able to spot.
"It's not just that the cancer is completely gone," de la Zerda says. "The most important thing is that we do not have to take huge amounts of healthy brain in the process. And so now we can actually imagine a world where surgeons, as they take away the tumor, actually know what to take out and they don't have to guess."
Next-generation imaging will help target cancer treatments
"Where medical imaging is heading to is the ability to look into the human body and actually see each and every one of these cells separately," de la Zerda says about his lab at Stanford. This would not only allow us to pick up tumors at a very early stage, it would also inform the right line of questions.
"In the lab we're now getting to a point where we can actually start asking these cancer cells real questions, like, for example, are you responding to the treatment we're giving you or not? So if you're not responding we will know to stop the treatment right away days into the treatment – not three months."
That means fewer patients suffering through the side effects of a chemotherapy drug that isn't even working, and more time on the clock to find a treatment that could help.
While that may not be outright victory in the war on cancer, it's an important weapon to add to our arsenal. "To be frank, we're still pretty far away from winning the war against cancer," de la Zerda says. "But at least I'm hopeful that we should be able to fight this war with better medical imaging techniques in a way that is not blind."

