
Photo by Rod Searcey: Jennifer Cochran and Amato Giaccia are members of a team of researchers who have developed an experimental therapy to treat metastatic cancer.
Stanford News - March 31st, 2016 - by Amy Adams
When new cancer cells break free of their original tumor, they travel the blood system and land in distant organs to kindle new tumors. It's these new cancer settlements, through a process called metastasis, that are often deadly.
One approach to preventing that spread has involved blocking the interaction of two proteins: one called Gas6 in the bloodstream and another called Axl that bristles along the outside of cancer cells. When these two interact, it signals the cell to pack up and go.
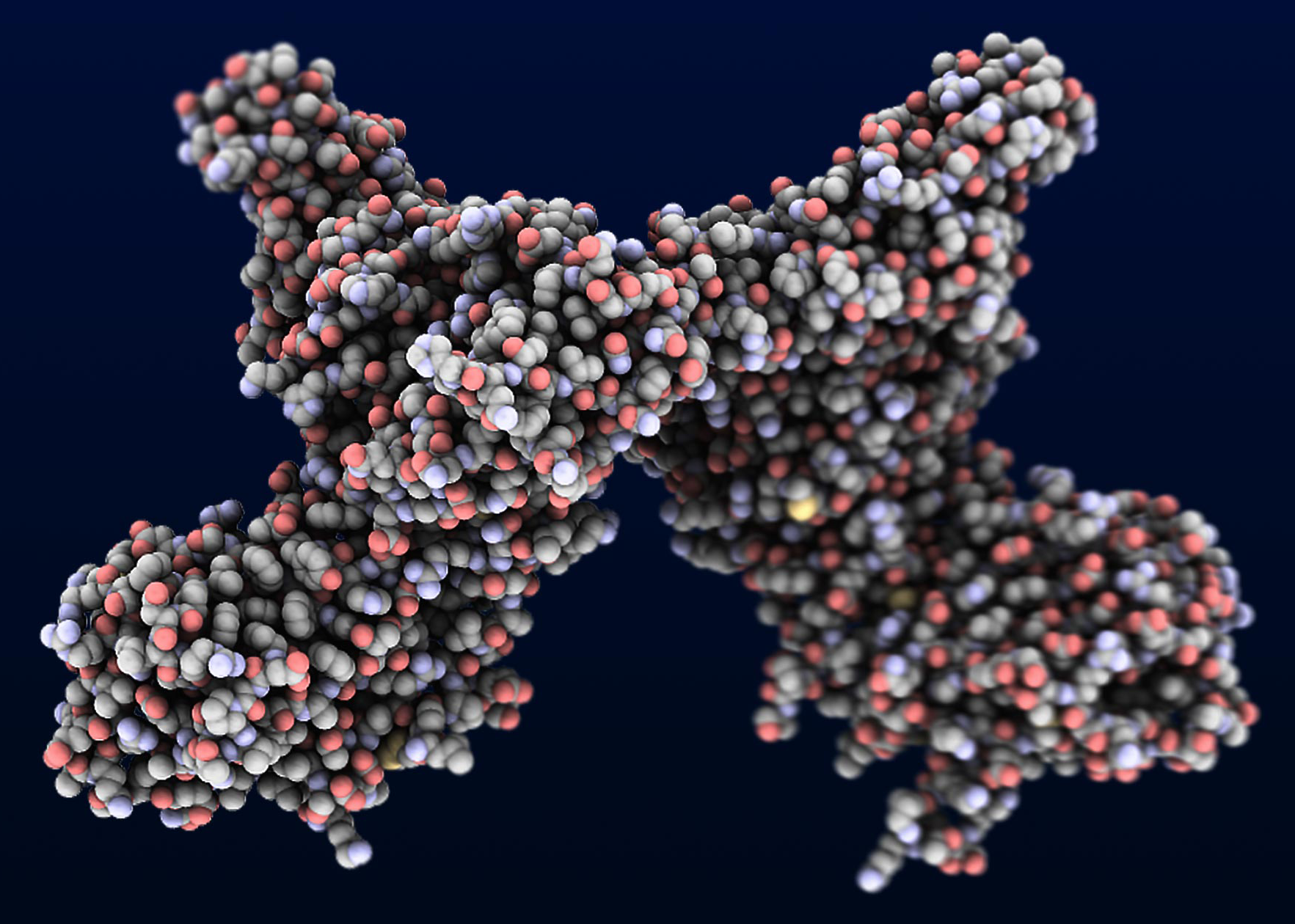
Illustration courtesy SLAC National Accelerator Laboratory:
A representation of the Axl protein reconstructed from X-ray
crystallography data.
Despite the promise, attempts at blocking that interaction haven't worked.
Jennifer Cochran, an associate professor of bioengineering, and Amato Giaccia, a professor of radiation oncology, thought they might try an alternate approach. Their idea was to create a decoy Axl protein that would latch onto Gas6 in the bloodstream, sopping up all the available Gas6 and preventing any from being available to bind to the real Axl.
To create that drug, the duo made more than 10 million mutations to the Axl protein, then tested those variants to find one that stuck most tightly to Gas6. One in particular stood out. It latched on to Gas6 much more tightly than the normal protein.
In mice, this Axl decoy protein when injected into the bloodstream sequesters the Gas6 and prevents metastasis in both breast and ovarian cancers. The team is now working to translate that success to people.
An additional question for Cochran was what changed in their mutated protein to make it so effective. "We wanted to understand the interaction on a deeper level," she said. "That curiosity is part of being an engineer."
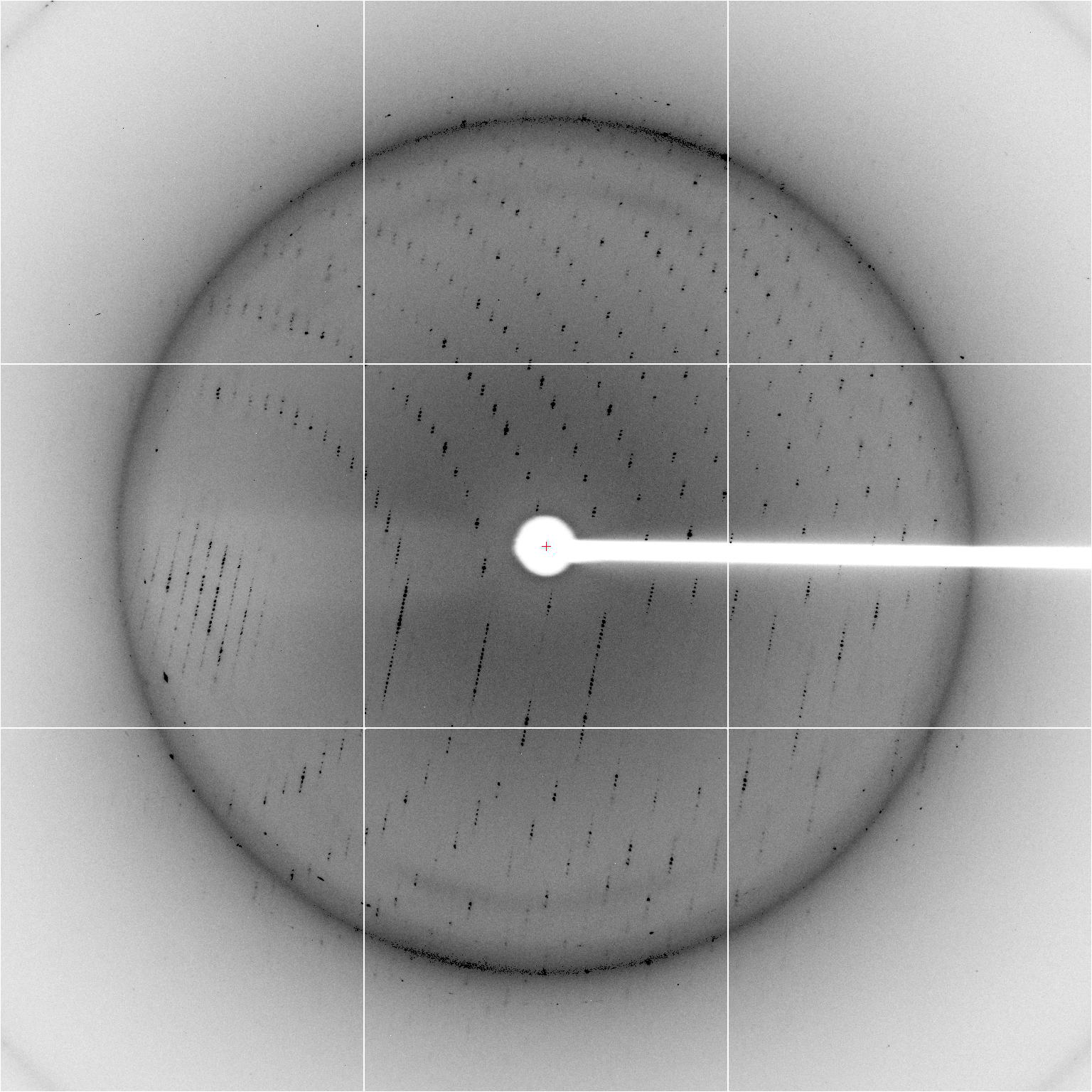
Image courtesy SLAC National Accelerator Laboratory: Pro-
tein crystal mounted on the X-Ray beam line with raw data
in the background. The data represents the organization of
atoms in the crystal.
Satisfying that curiosity could also help the team understand how the two proteins interact and predict ways of mutating other proteins to create drugs. "The idea is that if you could study this interaction you could use it in a predictive way down the road," she said.
Working with Irimpan Mathews, a structural biology scientist at SLAC's Stanford Synchotron Radiation Lightsource, Cochran and her team formed crystals of the mutant Axl interacting with Gas6. They then used a technique called X-ray crystallography, which essentially creates a molecular portrait of the proteins and their interactions.
"The crystallography revealed unique features that couldn't have been predicted," says Cochran, who is also a member of Stanford Bio-X. The tightest binding variant of Axl had a little pocket that helped it bind even tighter to the Gas6.
The Stanford-SLAC collaborators are now using similar methods to interfere with two proteins that help blood vessels infiltrate and support tumors, supported by a Stanford ChEM-H program to encourage Stanford faculty collaborations with SLAC.


