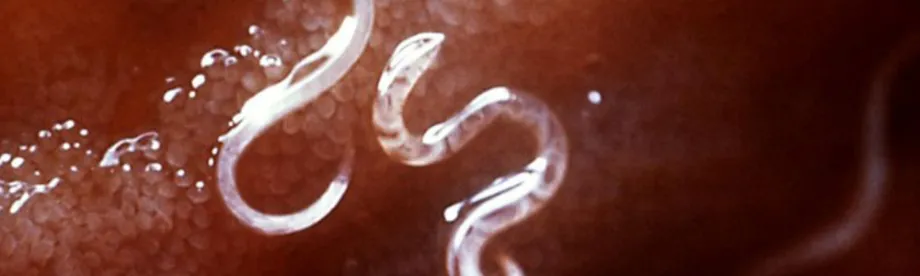
Photo from Wikipedia: Hookworms and other parasitic worms cause infections that claim the lives of 150,000 people each year. While most treatment efforts focus on children, a new study shows that extending treatment to adults is cost-effective.
Stanford Medicine News Center - September 16th, 2015 - by Tracie White
Stanford University School of Medicine researchers and their colleagues are calling for an urgent re-evaluation of global guidelines for the treatment of parasitic-worm diseases in light of a new study showing that large-scale treatment programs are highly cost-effective.
Parasitic-worm diseases afflict some 1.5 billion people in the developing world, causing gastrointestinal problems, anemia, wasting, and cognitive and growth deficits in children, and in some cases, liver, bladder and intestinal problems that can be fatal. About 150,000 people die of complications from these parasitic infections every year.
World Health Organization guidelines on treatment of the diseases focus only on school-aged children, as they are heavily affected by these diseases and can be easily treated in a school setting. The current guidelines call for annual or biennial treatment of children in high-prevalence areas.
But the latest study, a modeling analysis of four different communities in the Ivory Coast, suggests that more frequent, community-wide treatment programs are far more beneficial, both for children and adults, and are cost-effective.
“Most of the money spent on treating these diseases is focused on helping kids. But there are a lot of symptoms of disability in adults as well, and our results support the expansion of treatment to this adult population,” said Nathan Lo, a third-year Stanford medical student and research associate. Moreover, treating adults benefits children by reducing the chances they will become re-infected, he said.
“If you only treat children, it might help them, but they often come home to neighbors, parents and teachers who may be infected, and the children can once again become infected,” Lo said. “It’s more effective for children if you treat them and the people around them.”
Lo is lead author of the study, published online Sept. 15 in The Lancet Global Health.
Diseases prevalent in developing world
Jason Andrews, MD, the senior author of the study, said current global guidelines for mass drug administration for these infections have been based on expert opinion.
“Our approach was to look at this in a framework that considered the benefits of averting transmission along with morbidity, and to apply a rigorous cost-effectiveness model,” said Andrews, an assistant professor of medicine at Stanford. “We found that when you do so, the results strongly support a much broader treatment scope than has been historically recommended.”
Photo from Wikipedia: Schistosoma worms and other
parasites can be treated with drugs that are cheap and
widely available.
Parasitic worm diseases are among the most prevalent ailments in the developing world. They include helminth infections, such as roundworm, whipworm and hookworm, which are primarily found in soil. These parasites can slither around in the colon and small intestine, where they produce eggs that are passed in human feces and then spread through soil or water supplies. They are typically contracted by people who walk barefoot in an affected area or drink contaminated food and water.
Schistosoma is the other major category of parasitic worms, which tend to accumulate in the blood vessels around the bladder or intestines, sometimes migrating into the liver. These worms reproduce in waterborne snails and can be contracted by swimming in freshwater lakes or rivers or walking through contaminated, muddy fields.
The parasites can be readily treated with drugs that are cheap and widely available. Albendazole, which costs about 3 cents a pill, can reduce the number of worm eggs from the soil-transmitted helminths by as much as 95 percent, Lo said. Praziquantel, which costs about 21 cents a pill to administer, can reduce egg production by 98 percent in cases of schistosomiasis, which is a disease caused by the Schistosoma worms, he said.
In the study, the researchers looked at the value of these drug therapies in different settings, using data from four communities in the Ivory Coast between March 1997 and September 2010. They simulated different treatment scenarios in four 5,000-person communities, which had varying levels of risk for infection with soil-transmitted helminths and Schistosoma in the school-aged and adult populations.
Under current WHO treatment guidelines, three of the four communities would not have been eligible for annual treatment with both drugs because their risks were considered too low, the scientists noted.
The researchers looked at the impact when treatment was expanded to include preschool children and adults, and compared this to a scenario in which no treatment was offered. They also looked at variable timing of treatment — at intervals of three months, four months, six months, one year, two years, three years and four years.
They assumed a total treatment cost of 74 cents per person for school-based delivery and $1.74 per person for community-based delivery, with most of the expense involving drug delivery, staff and planning. Most drugs now are supplied in the developing world free of charge by pharmaceutical companies as part of their corporate global responsibility programs, though for purposes of the study, the researchers assumed the drugs were not donated, Lo said.
To calculate the impact of the various treatment scenarios, the researchers used a measurement known as the disability-adjusted life-year, a standard WHO measure to gauge the impact of disease.
Broader treatment would be cost-effective
They found that expanded, community-wide treatment programs were well worth the investment, with an incremental cost-effectiveness ratio of $167, meaning that it would cost $167 to save one year of a person’s life. By the standard health economics yardstick applied in the developing world, a treatment is considered highly cost-effective if it has an incremental cost-effectiveness ratio of less than $1,000, Lo said. By contrast, in the United States, that same cost-effectiveness threshold is $50,000, he said.
Even if treatment costs were much more than estimated in the study — as much as 10 times greater — the researchers found the treatment programs were still highly cost-effective. They also showed that even when treatment programs didn’t eliminate disease, they still had sufficient impact on health and disability to make them a worthwhile approach, Lo said.
“People may say, ‘Look, we’ve done mass drug administration, and the disease is still there,’” Lo said. “What we are showing is that even if the prevalence hasn’t changed, it’s likely you are averting symptoms and improving quality of life and curing people for some period of time. And it’s still highly cost-effective.”
The scientists also reported that treating people more frequently — at six-month intervals — was a more valuable approach to controlling the diseases. For instance, current WHO guidelines recommend annual treatment among school-aged children when at least 50 percent of them have schistosomiasis. Even in areas where prevalence is lower, more frequent treatment would be cost-effective and beneficial, the study found.
The scientists also found that treating the diseases together, rather than through separate programs, is a more efficient way to control these infections. “Most of the expense involved is in delivery, going out into communities and treating people,” Lo said. “So when these diseases are overlapping, as is often the case, it makes sense to give these pills together.”
Given the results, the researchers strongly urge WHO to reconsider its treatment guidelines to better manage these scourges.
“Revised guidance is urgently needed to inform the scale-up of treatment programmes worldwide to avert the substantial disability created from soil-transmitted helminthiasis, schistosomiasis and other neglected tropical diseases,” the study said.
The other Stanford-affiliated author on the study was Brian Blackburn, MD, clinical associate professor of medicine.
The study was funded by the Stanford School of Medicine’s Medical Scholars Research Program and by the Mount Sinai Hospital-University Health Network AMO Innovation Fund.
Stanford’s Department of Medicine also supported the work.

