
Image by K_E_N, Shutterstock.
Stanford News - March 11th, 2021 - by Holly Alyssa MacCormick
A little competition is never a bad thing, especially when it comes to fledging neurons growing in the brain, finds a new Stanford University study.
In a first of its kind study, researchers led by Stanford biologist Liqun Luo used genetic experiments and computer models to shed light on two important steps of brain development in young mice: the growth of branching extensions on the bodies of neurons, called dendrites, and the connections that dendrites make with other neurons. Like biological antennas, dendrites receive incoming signals from other neurons via connections called synapses. Luo’s team found that the dendrites of growing neurons compete with one another to form connections with their partners, and the presence of successful connections increases the odds of dendrite growth.
The findings, published in February in the journal Neuron, reveal that competitive interactions matter when neurons grow and form circuits. They also get at fundamental questions in neuroscience, said Luo, the Ann and Bill Swindells Professor in Stanford’s School of Humanities and Sciences.
“How does the brain get wired up? How do neural circuits form? These are big, unanswered questions,” Luo said.
The brain’s wiring
That’s because how neurons grow is a chicken-and-egg problem, explained Luo. Do dendrites have to exist before synapses can form? Or is the formation of synaptic connections vital for dendritic growth?
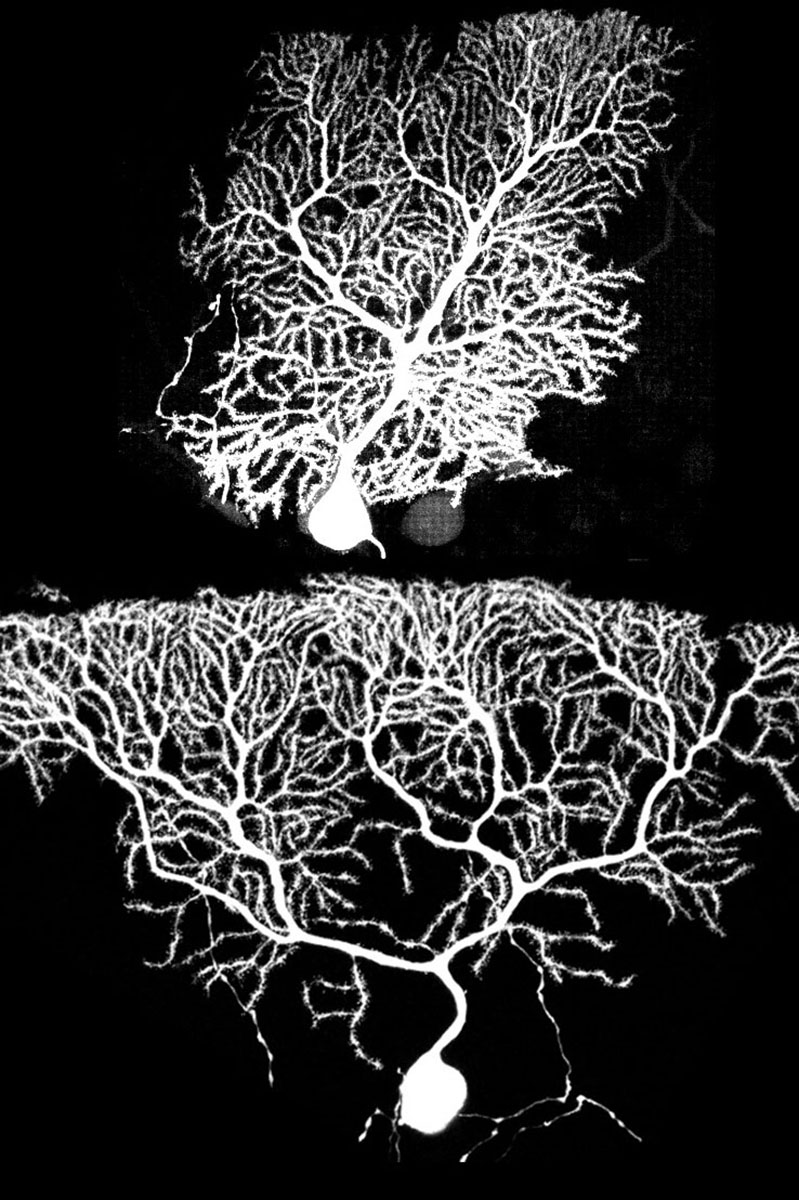
Image courtesy Andrew Shuster (click to enlarge): A normal
Purkinje brain cell (top) and a Purkinje cell with a GluD2 gene
mutation (bottom) that affects the ability to make early synaptic
connections with neighbors.
According to one idea, called the synaptotrophic hypothesis, synapses stabilize dendrites and make them more likely to grow further, while dendrites without synapses are more likely to recede. Luo said that, as far as he knew, this theory has never been tested in a developing mammalian brain before. So, he decided his lab would be the first to do so.
Luo’s lab specializes in exploring how neural circuits form during development, and how they’re organized to perform specific functions. For more than two decades his lab has investigated these questions, oftentimes using Purkinje cells, principal neurons in the cerebellum that influence motor and cognitive functions.
“Purkinje cells are my first love because they were the first mammalian neurons I studied, while I was still a postdoc,” Luo said. “They look like beautiful trees and there are many genetic tools to study them.”
Several of these genetic tools were developed in Luo’s lab, like the Mosaic Analysis with Double Marker (MADM) technique, which deletes a gene of interest from isolated single cells and labels those cells with a unique marker.
Imagine one Purkinje cell is a tree, Luo explained. “Labeling individual cells allows you to light up one whole tree in a dense forest of neurons, whereas if all Purkinje cells are labeled, it is difficult to visualize how an individual tree looks like.”
Growing Purkinje cells form circuits via synapses that are organized into layers, with a cerebellin-1 (Cbln1) molecule on one side and a glutamate receptor delta 2 (GluD2) protein on the other.
In the new study, Luo’s team used several different methods to alter the Cbln1 and GluD2 genes. They also used computer models that simulated dendritic growth and synapse formation to further explore their research questions.
In one experiment, the researchers manipulated Purkinje cells in developing mouse embryos using the MADM and other techniques to delete GluD2 gene and label the altered cells.
The researchers found that knocking out the GluD2 gene in all Purkinje cells had no noticeable effect on dendritic growth, but when only isolated Purkinje cells lost the GluD2 gene the results were striking.
Purkinje cells with the functioning GluD2 gene grew in their usual boxy shape with even dendrite branches at the tree’s bottom (early growth) and top (later growth). In contrast, Purkinje cells lacking the GluD2 gene had little dendrite growth early on, resulting in Purkinje cells shaped like upside-down pyramids.
“The key to this study is the ability to compare neighboring Purkinje cells that have and lack the GluD2 gene,” Luo said. “This reveals the competition among dendrites for synapses and how dendrites grow with normal or reduced synapses to stabilize them.”
The scientists were surprised by the upside-down pyramid shape of the Purkinje cells that lacked the GluD2 gene. “The lack of early dendrite growth was predicted by the synaptotrophic hypothesis, but the overgrowth of dendrites on top in late development was not expected,” Luo said. One possible explanation is that synapse formation may aid early, but inhibit late dendrite growth, he added.
The findings of this study bring Luo’s lab and the neuroscience community one step closer towards understanding how the brain is wired during development. “It’s basic science, but it also has implications for neurodevelopmental and psychiatric disorders,” Luo said.
This study was co-led by a visiting postdoctoral scientist Yukari Takeo and Neurosciences Program PhD student Andrew Shuster. The modeling was performed by Linnie Jiang, also a Neurosciences Program PhD student, during her first-year rotation, jointly supervised by Luo and Surya Ganguli, Associate Professor of Applied Physics.
The research was mainly supported by the National Institutes of Health and the Howard Hughes Medical Institute. Shuster and Jiang were also supported by Stanford graduate fellowships.


