Graphic by John Hersey.
Stanford Medicine Magazine - August, 2017 - by Ruthann Richter
Though Emery Olcott can no longer see words on a page or faces in front of him, he can still take pleasure in a game of golf. Legally blind, Olcott can’t drive a car, but occasionally he’ll pilot a golf cart on the course. And he can distinguish contrasts of light and color, like a small, yellow ball on an expanse of green.
Olcott has glaucoma, which gradually destroys the optic nerve, rendering people blind. The nerve is the bridge between the eye and the brain, and once it’s gone, it can’t be brought back. Or so the thinking goes. But scientists now are pushing the boundaries of biology and technology to revitalize the nerve. It’s one example of how researchers are working to help people regain or preserve their sight, seizing on new technologies and a better understanding of the eye.
It’s research that gives Olcott great hope. “I’m losing my vision, and I want to do anything I can to keep it,” says the 78-year-old retired businessman. “These guys are my lifeline now.”
The ability to see is one of our most precious assets, with polls showing that Americans fear losing their sight more than losing their hearing, speech or memory.
“Studies show that when it comes to their health, the thing people most worry about, after death, is losing their vision,” says Jeffrey Goldberg, MD, professor and chair of ophthalmology at Stanford. “People’s productivity and their activities of daily life hinge critically on vision, more than any other sense.”
Some 285 million people worldwide have limited or no vision, including 39 million who are blind, according to the World Health Organization. In the United States, some 36 million people suffer from vision loss or blindness, most commonly caused by glaucoma, cataracts, macular degeneration and diabetes-related complications, according to the federal Centers for Disease Control and Prevention.
Vision loss is one of the nation’s most costly health conditions, accounting for $139 billion in health expenses in 2013, according to Prevent Blindness America. It also can extract an emotional toll on patients, who may feel isolated and inadequate in not being able to fully care for themselves, Goldberg says.
“The fear of vision loss, even for people in lesser stages of disease, can be quite dramatic,” Goldberg says. “So anything we can do to stabilize, better diagnose and hopefully one day restore vision in some of these diseases I think will have an enormous global impact.”
The beauty of vision and how it works
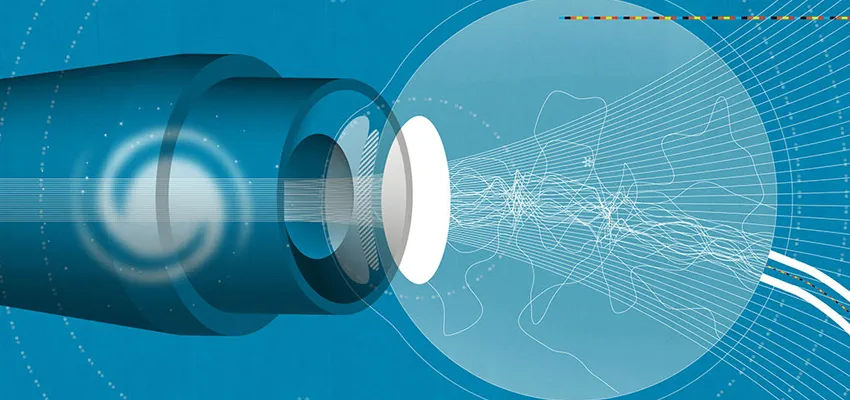
Of all the human senses, we rely most on our sight to understand and navigate the world around us, in all its color, texture and nuance. That’s why the eye is so extraordinarily complex, a powerful little sphere packed with more than 100 million neurons, able to transmit messages with split-second precision. Think of it as an extremely sophisticated camera.
Light first enters the eye through the cornea, the clear window that helps bring images into focus. The iris, the colored part of the eye, works like a camera shutter, adjusting in size according to the amount of light coming in. The lens, which is suspended behind the iris, fine-tunes the image focus, enabling a person to see things far away or up close.
The light then travels to the retina, the “thinking” part of the eye. It consists of a thin layer of millions of cells that coat the eye’s inner wall, among them more than 100 different kinds of nerve cells, each with its own job to do. The retina works like camera film to process the incoming information. It has some 120 million photoreceptor cells, specialized nerve cells known as rods and cones that are sensitive to light, and these enable us to see at night or in low light (rods) or to perceive color and fine detail (cones). The biggest concentration of cones is found in the macula, a small area in the center of the retina that allows us to read fine print, like the words in this story. The photoreceptors all relay their information to the retinal ganglion cells, whose long projections, known as axons, form the optic nerve. This bundle of a million fibers is the eye’s direct line to the brain, which translates it all into a meaningful image.
The retina and the optic nerve are formed in the embryo as part of the developing brain, says Michael Marmor, MD, professor and former chair of ophthalmology. “So retina really is brain. In a sense, we think inside our eye.” That’s because those 120 million photoreceptor cells that pick up an image have only 1 million optic nerve fibers to carry their electrical signals along. “So the retina has to code the information and simplify it. We do not send pictures to the brain. We send code that highlights the important things.”
Of course, things can go wrong at any step of the process to compromise or completely obscure our sight. For one, the cornea has to be kept moist and clear and can be damaged by trauma, infection or dryness. The eye is also subject to the rigors of aging. The lens in the eye may become clouded with age, leading to cataracts, which can be readily corrected with surgery. The macula also may deteriorate over time, leading to gaps that may limit vision, or fail it altogether; treatments to intervene in the retina are limited and not entirely effective.
As a fluid-filled sphere, the eye also needs to maintain a certain level of pressure to keep its shape. Without that, it may collapse, like an empty balloon, Marmor says. That pressure is being constantly regulated, but sometimes the regulatory system fails, and fluid builds up along with pressure in the eye. That can damage the optic nerve, leading to glaucoma and permanent vision loss. It’s the most common cause of irreversible vision loss — a calculus scientists hope to change.
Fixing the cornea with cell therapy and magnets
The cornea is particularly vulnerable to assault, as it is the eye’s entrée to the outside world. Seriously damaged corneas can be replaced with transplanted organs, but the transplants have big challenges: They rely on corneas from deceased donors, which are in scarce supply, and they fail as much as 30 percent of the time, depending on the disease, Goldberg says. Moreover, in the developing world, it’s a rare patient who has the option of a transplant.

Photo by Brian Smale: Goldberg is testing an implant in
the eye to treat glaucoma, the world’s No. 1 cause of
irreversible blindness.
Five years ago, Goldberg was trying out a new cornea transplant procedure in the operating room, hoping for a better success rate, when he suddenly hit upon an idea: What if patients could avoid the surgery altogether, if clinicians used replacement corneal cells and positioned them using tiny magnets?
The idea flowed from his laboratory experiments with magnetic nanoparticles, tiny spheres that can be manipulated with magnets. These particles are minute, just 50 nanometers in diameter, thousands of times smaller than a human hair.
“I thought, if we took these nanoparticles we were studying in the laboratory and loaded them into corneal endothelial cells, maybe we could deliver them into the eye with a magnet, without having to do surgery at all,” he said. “It was one ‘aha’ moment that I can really point to in my scientific career.”
His research team began using corneal endothelial cells from organ donors’ corneas (the same cells being used in surgical transplants), peeling off a layer of these cells from the cornea and then putting them immediately into laboratory dishes, where they bathed in growth factors. There they blossomed into tens of millions of new, young corneal cells, Goldberg says.
He attached these cells to iron oxide nanoparticles with a protective coating to prevent the iron from leaching out. Goldberg injected them into the eye with a tiny needle and used a magnetic patch to pull them up into the inside of the corneas of patients.
The new cells “put out their elbows, and nuzzle into the tissue, and that’s where they stay and function,” he says. Later, the magnetic particles fall off the cells and are excreted from the body.
In the initial safety trial last year, his collaborators at the Association to Avoid Blindness, a major eye hospital in Mexico City, tested the treatment in 10 patients. They began with seven patients with very advanced disease, but as it became apparent that the treatment was safe, they moved on to three other patients with somewhat less-serious damage. One patient, who had started the trial with 20/200 vision, which is considered legally blind, left the trial with 20/40 vision. “It was quite dramatic,” Goldberg says. “So we’re very excited about it.”
He and his colleagues plan to apply this fall to the U.S. Food and Drug Administration to test the procedure’s safety and efficacy in up to 60 patients at several U.S. medical centers, including Stanford. If it works, the procedure could replace certain transplant surgeries, some 100,000 of which are performed every year in the country. It also could be used to treat a variety of corneal conditions, such as Fuchs’ endothelial dystrophy (corneal damage caused by a buildup of fluid) and pseudophakic bullous keratopathy (the breakdown of corneal cells after cataract surgery), he says.
Goldberg also has a grant from the California Institute for Regenerative Medicine to see if it’s possible to turn pluripotent stem cells into corneal endothelial cells, bypassing the need for donor cells completely.
“The idea is that instead of growing up tens of millions of cells, if we could do it from stem cells, we could grow a billion cells and have a freezer full of eye treatments,” he says.
Mending the retina
Daniel Palanker, PhD, resolved to find new ways to repair the retina after a laboratory mishap that could have cost him his own sight. A physicist by training, he had been experimenting with an ultrafast laser in 1998 when he fell forward, his eye briefly crossing the high-intensity beam. He knew instantly that he was in trouble — that the laser could have cut his retina.
“I knew exactly what was happening because I was studying laser-tissue interactions, and when I heard a high-pitched buzz, I knew it was cutting tissue. There was bleeding,” Palanker, then a Stanford postdoctoral scholar, recalled recently.
In the emergency room at Stanford Hospital, he struck up a conversation with Marmor, his treating physician. “I had some ideas from my PhD work about retinal laser therapy and I asked if there was someone I could talk to about applications of lasers. He said, ‘Yes, we just got a new chair who is a retinal surgeon, why don’t you talk to him?’”
As it happened, the new chair, Mark Blumenkranz, MD, had read some of Palanker’s papers and was very keen on his ideas. Though Palanker was just two weeks away from leaving Stanford for a faculty position at Harvard, Blumenkranz persuaded him to stay and help build Stanford’s budding research program in ophthalmology.
Even though his own injury healed, he began his quest for new treatments and technologies to fix retinal problems. He went on to start multiple companies with Blumenkranz and invent the PASCAL laser — the tool of choice in retinal laser treatment today, used for diabetes-related eye problems and other conditions. He also invented the femtosecond laser system for cataract surgery and a neural stimulator for dry eye therapy, among other technologies.
Most recently, Palanker has devised a new laser treatment that he hopes will be effective in preventing macular degeneration, a progressive condition that is common among older adults and is considered incurable. The new, non-damaging laser therapy works by applying a low level of heat, enough to stimulate retinal cells without causing them harm.
The laser is absorbed primarily in the retinal pigment epithelium, a nourishing layer of cells that recycle protein debris produced by photoreceptors. With age, sometimes these cells fail in the job, allowing toxic garbage to pile up. Eventually the garbage may suffocate and kill the photoreceptors, severely impairing patients’ central vision. The laser therapy aims to rejuvenate the retinal pigment epithelial cells so they can effectively do their job and keep photoreceptors alive, says Palanker, now a professor of ophthalmology and director of the Hansen Experimental Physics Laboratory at Stanford.
“After thermal stress, cells undergo rejuvenation and become more metabolically active,” he says. “The hope here is that they will actually recycle the garbage and thereby reset the clock, so to speak.”
In a pilot study, Daniel Lavinsky, MD, a former postdoctoral scholar in Palanker’s lab, tested the treatment in 20 patients in Brazil with central serous chorioretinopathy, in which fluid leaking under the retina leads to blurred vision. The non-damaging laser therapy completely cleared out the fluid in more than 80 percent of the cases and reduced the fluid in the rest of the eyes, he and his colleagues reported in May 2016 in Investigative Ophthalmology & Visual Science.
The researchers also tested the treatment in 10 patients with an intractable eye problem known as macular telangiectasia, and they responded very well.
“We are actually causing the cells to self-repair,” says Lavinsky, now a professor of ophthalmology at the Federal University of Rio Grande do Sul in Brazil. “Instead of damaging cells, as was done previously with conventional laser treatment, we are trying to maintain their integrity and enhance their rate of function.”
He and his colleagues now are testing it as a way to prevent macular degeneration, on the assumption that it could help rejuvenate retinal epithelium cells in the macula. They have begun a two-year trial at Stanford and at the Bascom Palmer Eye Institute in Miami among 56 men and women with early stages of the disease, in the hope that it will stop its progression. Typically, patients develop little yellow spots on the retina, deposits of uncollected protein garbage known as drusen. The researchers will monitor patients to see if the drusen shrink and disappear, a sign the treatment is working.
A consortium of ophthalmologists is also planning to test the therapy in two larger trials, one involving diabetic patients with macular edema, or fluid accumulation in the retina, and another in patients with macular telangiectasia, Palanker says.
“The treatment itself is very easy and painless,” he says. “The heat level is very low, so it’s not killing cells. If it doesn’t work, you have nothing to lose — it’s not damaging anything.”
The retina also often becomes impaired in people with diabetes, particularly if their blood sugar levels are not well-controlled. This condition, known as diabetic retinopathy, is the most common cause of blindness in the United States, affecting 7.7 million people over age 40 — yet it remains poorly understood, says Sui Wang, PhD, assistant professor of ophthalmology.
The disease is insidious, beginning when high sugar levels cause blood vessels in the retina to weaken, becoming leaky and forming tiny bulges known as aneurysms. Wang says patients don’t notice changes in their eyes until the disease enters a more dangerous state, with blood vessels growing out of control and ultimately blocking the retina.
She says it’s believed that excess sugar triggers inflammation and oxidative stress in retinal cells, though the mechanisms aren’t known. In studies with rodents, she is looking at how blood sugar impacts different types of retinal cells at the molecular level and what specific genes and pathways may be involved.
“If we can figure out those pathways,” she says, “maybe we can prevent it from going to the second stage” and preserve patients’ sight. She is also using genetic analysis and techniques to manipulate genes in the retina with the goal of developing new drugs or gene therapies that could protect the retina from diabetes or even restore vision in those already suffering eye damage from the disease.
Fixing the optic nerve — can it be done?
For Olcott, the first sign of eye trouble surfaced during a routine eye exam when he was in his mid-30s. The clinician noticed his eye pressure was unusually high — a risk factor for glaucoma. But Olcott didn’t realize then that his eyesight might be under threat.
“With glaucoma, you will find you never know you have it,” he says. “You don’t feel the pressure. It’s one of those silent killers.”
As his vision declined, he had multiple procedures to relieve the pressure, with doctors creating a hole to release some of the fluid. But these measures are only a temporary fix, medicine’s inadequate answer to the disease. So five years ago, he signed up for a procedure Goldberg was just beginning to study in patients, a surgical implant to help protect and regrow nerve cells damaged by glaucoma and other optic nerve diseases.
The implant, made by Neurotech, contains human cells genetically engineered to pump out ciliary neurotrophic factor. First isolated in the 1980s, this naturally occurring growth factor has been shown in many animal studies to protect and regenerate axons in the optic nerve.
Goldberg, then at the Bascom Palmer Eye Institute in Miami, began by testing the implant in 11 patients with glaucoma and 11 with a blocked blood vessel in the optic nerve, called ischemic optic neuropathy. He inserted the 1 millimeter by 5 millimeter capsule through the white part of the eye, where it began releasing a steady flow of CNTF from nourishing cells packed inside. These cells feed the retinal ganglion cells, giving them a kind of “booster shot to stay healthy or improve vision,” he says. A semipermeable membrane keeps the cells inside the capsule in the eye, so they can’t be targeted for rejection by the immune system. The implant is being tested at multiple sites around the country.
“It’s a completely unique approach to attacking the problem of glaucoma,” says Joel Schuman, MD, professor and chair of ophthalmology at NYU and a glaucoma specialist. “The reason is that it is enhancing the function of neurons that are in existence but not functioning at the top of their ability.”
Olcott had the implant installed in his right eye, recalling no pain or discomfort during or after the process. “It stabilized the eye, which didn’t deteriorate as much as they thought it would have otherwise,” he says. It is now his better eye, as his left eye has deteriorated and completely lost its power.
Goldberg and his collaborators are now testing the device in 60 glaucoma patients to see if it can stabilize or reverse their vision loss.
“Glaucoma has always been touted as the No. 1 cause of irreversible blindness in the world,” he says, affecting 6 percent of older adults in the United States. “But that doesn’t have to be the case. We can apply the therapy and see if we can reverse vision loss. That’s the advantage of taking some of these findings in the lab and testing if we can use them as effective treatments.”
Goldberg is also working closely with Andrew Huberman, PhD, in a clinical trial that uses a form of visual stimulation to revitalize dormant retinal cells with the goal of helping glaucoma patients retain their sight. In the therapy, patients will be immersed in a virtual reality environment — a virtual tour of an art gallery — that exercises their eyes and provides visual interest to keep them engaged. The therapy can’t bring back to life cells that have died but it may be able to revive those that are working poorly.
“You might not be able to take people who are completely blind and make them see,” says Huberman, an associate professor of neurobiology at Stanford. “But you could imagine taking people who are losing their vision and helping them hold on to what they have. That would be really exciting because a lot of what glaucoma patients fear is losing what little vision they have, like people in the early stage of Alzheimer’s worry about losing their mind.”
The project grew out of experiments by Huberman and his colleagues that coaxed mouse retinal ganglion cells back to life through visual stimulation and biochemical manipulation — essentially re-creating what happens in the developing brain, when immature neurons produce growth-promoting molecules to encourage new neurons to thrive and form visual circuits.
“All you have to do is trick the neurons into thinking they are baby neurons again,” he says. “That is basically what we did.”
Remarkably, the combination therapy spurred retinal ganglion cells to sprout new axons — critical connectors to the brain. Most importantly, the axons grew back through the eye and into the brain, where they linked up in all the right places — something that had never been observed before. The mice didn’t regain perfect sight but could see large objects coming so they could dart out of the way.
“We found the wiring was highly precise,” Huberman says. “That means these neurons know how to find their way home.”
Goldberg has also had some success in repairing the optic nerve in animals, using a form of cell replacement. He and his colleagues transplanted retinal ganglion cells from newborn mice into the retinas of adult rats. They used fluorescent tags to track the cells and found they behaved much like native cells, growing axons and forming connections that reached their intended targets within the brain. The study was published in February 2016 in Nature Communications.
Huberman’s study, which appeared in July 2016 in Nature Neuroscience, attracted much media and public attention — so much so that he was besieged by thousands of emails from patients, eager to know when he was going to try the therapy in people, he says. He decided to move into human testing with a trial that has been approved by Stanford’s Institutional Review Board and is likely to start this fall.
Here is how it works:
Patients will don a pair of virtual reality goggles that take them into an alternate reality — in this case a museum gallery of 11 master artworks hidden behind empty frames. With a press of the thumb, they can select a frame and watch it slowly come to life, revealing such classics as Edvard Munch’s “The Scream” and Leonardo da Vinci’s “Mona Lisa.”
The paintings are their visual reward, a motivation to stay with the program. But first their eyes get some exercise — a series of flashing dots stimulate them.
“All these dots are triggering the ganglion cells to fire,” says Bireswar Laha, PhD, a postdoctoral scholar in Huberman’s lab, as he demonstrates the program. The dots flash in specific patterns, designed to stimulate specific types of ganglion cells, he says.
Patients will be able to do the therapy at home, browsing the gallery for 30 minutes a day over the course of a month or two. Each week, they will be tested in the clinic to see if the stimulation has had an impact on their sight. Huberman says the therapy isn’t likely to help someone who has no ganglion cells at all and is completely blind. But it could stimulate new axons to grow in ganglion cells that are still alive and have the capacity to reach out to the brain.
“Take someone with huge holes in their visual field — someone who is going blind, who can’t read or drive or see their children. Can we bring back some vision? Maybe,” he says. “We can exercise the ganglion cells and that might let them hold on to what they have.”
The technology is simple and affordable, requiring just a computer laptop and a pair of goggles, so it could be used in developing countries, where glaucoma affects some 60 million people. Many of these patients have no access to treatment and face the prospect of going blind.
“It’s inexpensive enough that you could imagine creating a whole, portable eye clinic for $1,000 that many patients could use,” Huberman says.
He says the trial will enroll up to 200 patients and is likely to last a year. Olcott is already vying for one of those slots.
“It could be transformative for me,” he says. “I’m keeping my fingers crossed because it involves a noninvasive technique aiming to help the optic nerve and brain make a better connection so a damaged optic nerve can communicate better with the brain. I’m pinning my hopes on that.”





