Interdisciplinary Initiatives Program Round 7 - 2014
Frederick Chin, Radiology
Edward Graves, Radiation Oncology
J. Martin Brown, Radiation Oncology
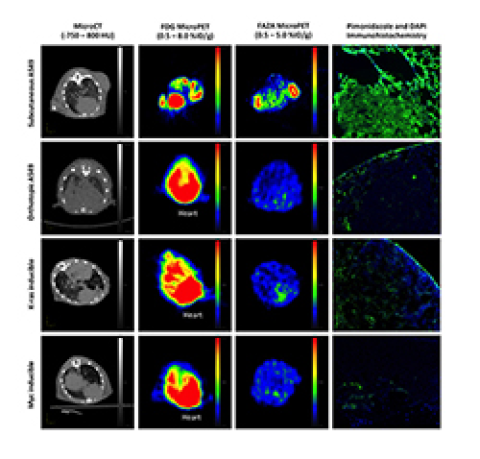
Tumor hypoxia (reduced oxygen concentration) has been associated with tumor aggressiveness and promotes broad resistance to radiotherapy and drug therapies. Useful imaging techniques could help provide accurate prognosis, stratify patients to determine the most effective therapy to pursue, and predict or monitor tumor response to specific therapies. The determination of the presence or absence of tumor hypoxia is important in planning hypoxia-selective treatments and avoidance of oxygen-dependent treatments. Molecular imaging with hypoxia-specific tracers should play an important role in selecting patients who might benefit from specific treatments. All current PET hypoxia-"specific" radiotracers, nitroimidazoles including [F-18]FMISO, [F-18]FAZA, [F-18]EF-5 and [Cu-64]ATSM (labeled with either radioactive fluorine-18 or copper-64), generate a positron emission tomography (PET) image based fundamentally on the signal intensity produced relative to the level of intracellular reductase activity under hypoxic conditions. A major disadvantage of these radiotracers is that tracer reoxidation can occur upon re-oxygenation of the tumor environment, which allows the tracer to subsequently escape from the tumor cell. Consequently, slow accumulation of these classic imaging agents into hypoxic tumors, and the escape of radioactive byproducts of these probes from the same tumor cells, results in a low target-to-background ratio for the corresponding PET images. Hence, our “trigger and release” strategy is designed to be the first truly specific approach to imaging tumor hypoxia. We propose an entirely novel, non-nitroimidazole, mechanism-based “trigger and release” strategy for imaging hypoxia with PET.
By combining the specialized expertise in the Frederick T. Chin (Radiochemistry/Molecular Imaging), Edward E. Graves (Lung Tumor Hypoxia/Radiotherapy), and J. Martin Brown (Brain Tumor Hypoxia/Radiotherapy) laboratories, a new tactical approach to imaging probe design, featuring a chemical “trigger” that causes a favorable “release” of the radiolabeled PET imaging agent, can be explored in tumor hypoxia models. There are many choices of fluorine-18-labeled molecules that we can design to be released and subsequently “trapped” inside the tumor cell under hypoxic conditions. The trigger of our “smart probe” can sense hypoxic tumor environments, and is mechanistically detached from the fluorine-18-bearing group within the tumor cell following a specific chemical step. The fate of the remaining non-radioactive trigger of our smart probe, in extremely low concentrations, is essentially invisible to PET radiation detectors and does not interfere with PET imaging. Under normal oxygen (normoxic) conditions, the smart probe is not triggered and thus, it is allowed to leave the cells and return to circulatory system. Uptake of our smart probe by normoxic cells should not result in release of the radiolabeled part of the molecule, ultimately providing improved contrast ratio between hypoxic and normoxic cells. This novel approach is highly flexible and offers a wide variety of triggers, linkers, and chemical side-products to fine-tune our strategy to achieve optimal imaging characteristics. Thus, this new and effective strategy for imaging hypoxia would empower the medical community to correctly identify cohorts of patients who may respond better to treatments designed to overcome the cure limitations of hypoxic tumors, to demarcate hypoxic tumor areas and adjust radiotherapy accordingly, and to properly characterize tumors in support of personalized medicine.