Photo by Juan Gaertner, Shutterstock.
Stanford Medicine Scope - August 16th, 2017 - by Bruce Goldman
In a new study in Neuron, Stanford researchers have used a revolutionary 3-D culture technique to nurse a very slowly developing set of brain cells known as astrocytes to maturity in laboratory glassware.
I recently wrote about a study, published in Nature, in which Stanford neuroscientist Sergiu Pasca, MD, and his colleagues showed the power of a method pioneered in Pasca's lab for growing brain cells in three-dimensional culture. This newfound ability to generate so-called "neural spheroids" -- almost perfectly round balls approaching 1/16 of an inch in diameter and consisting of over 1 million cells each -- recapitulates many aspects of the human forebrain's natural development. This in turn lets scientists study events that, in living humans, couldn't otherwise be monitored, much less manipulated, in order to learn about how the developing brain wires itself up and what happens when the wiring process goes wrong.
Pasca's neural spheroids accurately mirror the three-dimensional structure of the cerebral cortex’s six-layer-thick architecture. They contain all the subtypes of nerve cells (or, in science-speak, neurons) one would expect to see there, as well as working connections between them. Critically, they remain alive, viable and happy in laboratory glassware for more than two years, far longer than has been achieved previously.
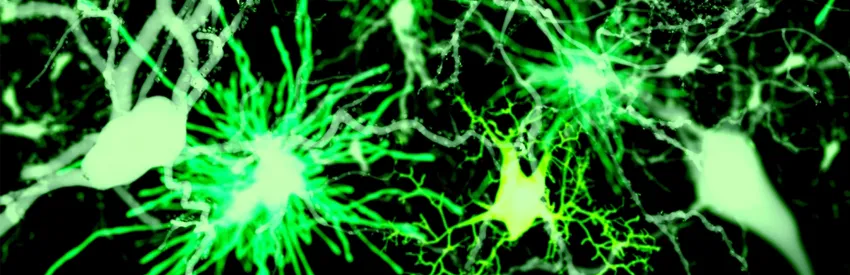
And just like real brains, neural spheroids also contain a crucial class of understudied brain cells -- astrocytes, which are not nerve cells, are absolutely critical to healthy brain function, and mature so slowly that previous culture methods have been unable to capture these cells' full developmental arc.
Several years ago, I wrote an article for our magazine, Stanford Medicine, about the little-appreciated fact that most of the brain’s cells aren’t nerve cells at all, and the even less-appreciated fact that these other cell types are key players in all kinds of operations that are critical to neural function. That article, titled “The Brain’s Silent Majority,” highlighted the pioneering work of Stanford neurobiologist Ben Barres, MD, PhD.
These non-nerve brain cells, around which Barres has built an illustrious career, include a category of star-shaped cells, aptly named astrocytes. These cells -- the most common in the human brain -- are stellar in more ways than one. They manage many of the second-to-second logistical needs of neurons. They help maintain the blood-brain barrier. They actively participate in forging and breathing life into synapses, the tiny, complicated connections neurons sprout to transmit information-packed impulses to one another. When synapses outlive their use or pose a risk of interfering with a brain circuit's current function, astrocytes assist in the pruning process by which these connections are erased.
Most recently, Barres and his co-workers have found that under certain conditions, astrocytes can go on a rampage and destroy the very neuronal tissue they ordinarily nurture so carefully, possibly looming large in the etiology of numerous neurodegenerative diseases.
In the new Neuron study, Barres and Pasca showed that Pasca's neural spheroids, which contain astrocytes, reliably replicate these cells' development of as well as that of neurons.
"Astrocytes' development in humans is very slow, and you can't accelerate it." Pasca told me. "Their maturation continues throughout infancy." The ability to study these cells' full development in culture portends advances in understanding neurodevelopmental disorders such as autism and schizophrenia -- without using fetal brain tissue.




