Photo by Timothy Archibald.
Stanford Medicine News Center - April 24, 2024 - by Bruce Goldman, Erin Digitale
For a long time, no one understood that Holden Hulet was having seizures.
“He would just say ‘I feel tingly, and my vision kind of goes blurry,’” said Holden’s mom, JJ Hulet. “But he couldn’t communicate exactly what was going on.”
JJ and Kelby Hulet could see their son was having short spells of incoherent speech, rapid back-and-forth eye movements and odd physical changes. “He’d kind of go — I don’t want to say ‘limp’ because he would stand just fine — but his body would just be in zombie mode,” JJ said. The episodes lasted less than a minute.

Read more in Nature!
The parents were puzzled and worried, as they had been many times since Holden was born in 2008 and they learned that their newborn had an extremely rare genetic disease. “I was thinking it was his heart,” Kelby Hulet, Holden’s dad, said.
Holden’s condition, Timothy syndrome, causes long, irregular gaps in heart rhythm. He spent his first six months hospitalized in a neonatal intensive care unit in his family’s home state of Utah while he grew big enough to receive an implantable cardioverter defibrillator. The device sends an electrical signal to restart his heart when it pauses for too long.
As a small child, Holden would sometimes pass out before the defibrillator shocked his heart back into action. When Holden started telling his parents about the blurry-vision episodes at age 6, Kelby initially believed it was a new version of the same problem, and he kept a time stamp on his phone for each episode. But the records from Holden’s defibrillator showed that these times did not line up with any heart-rhythm problems.
The family’s pediatrician was confused, too. Perhaps Holden was having periods of low blood sugar, another possible Timothy syndrome complication, he suggested. Initial testing at the local medical center did not turn up clear answers.
But Kelby, who was training to become an operating room nurse, realized Holden’s episodes reminded him of what he was learning about warning signs for stroke. JJ called Holden’s cardiologist in Utah and asked for a detailed neurologic evaluation, which enabled the mysterious episodes to be diagnosed as seizures. Holden began taking anti-seizure medication, which helped, to his parents’ great relief.
Researching a rare disease
A few months after Holden was born, Sergiu Pasca, MD, arrived at Stanford Medicine to pursue a postdoctoral fellowship in the lab of Ricardo Dolmetsch, PhD, then an assistant professor of neurobiology, who was redirecting his research to autism spectrum disorder. At the time, Pasca did not know the Hulet family. But his work soon became focused on the disorder that has shaped Holden’s life.
Caused by a defective gene on the 12th chromosome, Timothy syndrome is vanishingly rare, with no more than 70 diagnosed cases. Children with this disorder rarely survive to late adolescence. It is caused by a mutation in the gene coding for a type of calcium channel — a protein containing a pore that selectively opens or closes, respectively permitting or blocking the flow of calcium across cells’ membranes. While a prominent feature — severe heart malfunction — can be tackled with a pacemaker, most children with Timothy syndrome will end up with lifelong brain disorders including autism, epilepsy and schizophrenia.
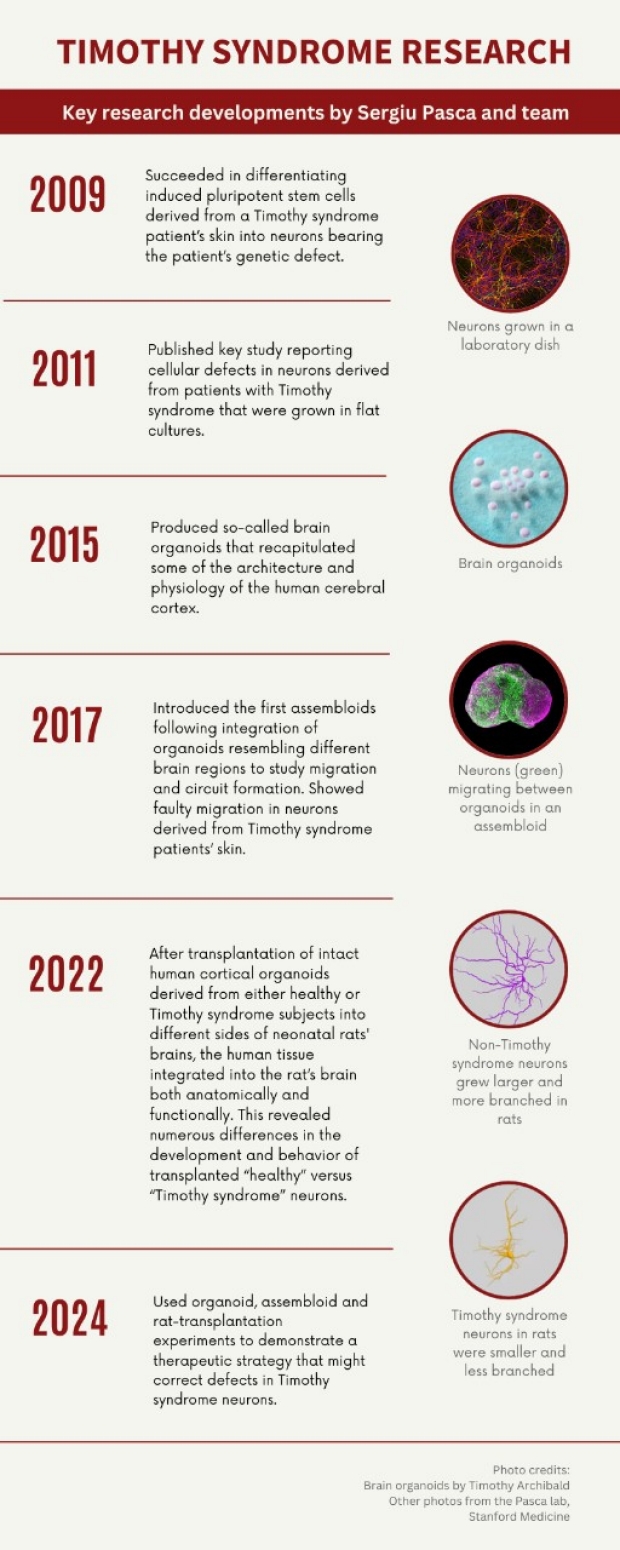
By mid-2009, Pasca had succeeded in generating nerve cells from induced pluripotent stem cells (which can be induced to form virtually any of the body’s numerous cell types). These included cells derived from the skin of two patients with Timothy syndrome. Later that year he observed defects in how the patient-derived neurons were handling calcium. This advance — the creation of one of the initial in-a-dish models of brain disease, built from neurons with defects that precisely mirrored those of a patient’s brain — was published in Nature Medicine in 2011.
Pasca and colleagues continued to monitor these Timothy-syndrome neurons in standard two-dimensional culture — growing as single layers in petri dishes — over the next few years. While this two-dimensional culture method was limited in its ability to sustain viable neurons, it was soon superseded by a genuine scientific breakthrough.
Pioneering the first assembloids
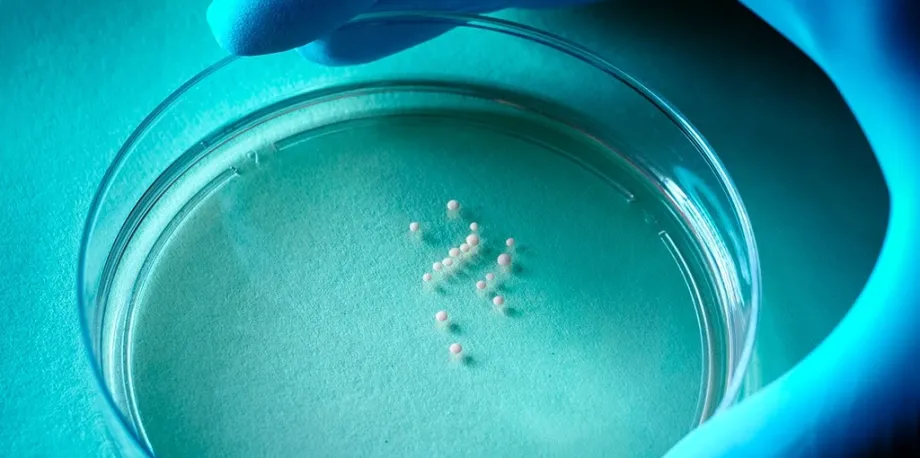
The constraints of two-dimensional culture, including the inability to keep these neurons for long periods of time so that they could reach key stages of neural development, prompted Pasca in 2011 to start developing an unprecedented three-dimensional method. The novel technology produced what came to be known as brain organoids. These constructs recapitulated some of the architecture and physiology of the human cerebral cortex. The organoids can survive for several years in culture, enabling neuroscientists to view, non-invasively, the developing human brain up close and in real time. The scientists wrote a seminal Nature Methods paper, published in 2015, that described their discovery.
Pasca’s group subsequently showed that culturing brain organoids in different ways could generate organoids representing different brain regions (in this case, the cerebral cortex and a fetal structure called the subpallium). In a breakthrough set of experiments, Pasca’s team found ways to bring these organoids into contact so that they fuse and forge complex neuronal connections mimicking those that arise during natural fetal and neonatal development. Pasca named such constructs assembloids.
In their paper on the research, which was published in Nature in 2017, Pasca’s team showed that after fusion, a class of inhibitory neurons originating in the subpallium migrates to the cortex, proceeding in discrete, stuttering jumps. (See animation.) These migrating neurons, called interneurons, upon reaching their destinations — excitatory neurons of the cortex — form complex circuits with those cortical neurons.
But in assembloids derived from Timothy syndrome patients, the motion of interneurons as they migrate from the subpallium is impaired — they jump forward more often, but each jump is considerably shorter, so they fail to integrate into the appropriate circuitry in the cortex. This wreaks havoc with signaling in cortical circuits. Pasca’s team tied this aberrant neuronal behavior on the part of Timothy syndrome neurons to the key molecular consequence of the genetic defect responsible for the condition: namely, malfunction of the critical channels through which calcium must pass to cross neurons’ outer membranes.
A family’s struggles
While Pasca was developing assembloids, the Hulet family was progressing through their own journey of discovery with Holden. They faced painful uncertainty at every stage, starting when Holden was discharged from the NICU in the summer of 2009, after several months of hospitalization and multiple heart surgeries.

Courtesy of the Hulet family: In this 2019 photo, Timothy syndrome patient Holden Hulet, left, rides in a side-by-side ATV driven by his dad, Kelby Hulet, at sand dunes near their home in southern Utah.
“Even when we brought him home, [his doctors] said ‘Don’t get your hopes up. We don’t usually see them make it past age 2,” JJ recalled. Many children with Timothy syndrome die from cardiac failure in early life.
“It’s really hard to be positive in that kind of situation, and for a long time I did let it get to me,” JJ said. “I finally got to a point where I said, ‘I have to live my life and we just keep fighting.’”
JJ runs a child care center and has years of experience working with special-needs kids, which motivated her to push for an autism evaluation when she saw signs of autism in Holden. He’s much more verbal than many children with autism, which paradoxically made it more difficult to get an official diagnosis.
“That was frustrating,” JJ said. Although the family’s pediatric cardiologist in Salt Lake City was familiar with the vagaries of Timothy syndrome, their local caregivers in the small town where they live in southern Utah were not. “They kept saying ‘Oh, no, it’s just developmental delays because he was so premature,’” she said. She wonders whether it would have been easier to have Holden’s autism diagnosed had more been known about Timothy syndrome at the time.
“I think research is important so that parents and children have the support they need,” she said, noting how lonely and painful it can be to advocate for a child when his condition is poorly understood — and when, as a parent, you may be doubted by medical professionals. “It’s a really hard thing to deal with.”
Her voice breaks briefly. She continues, “I think research brings validity to that.”
Implanting organoids
In 2022, Pasca published a study in Nature describing the transplantation of human cortical organoids into neonatal rats’ brains, which resulted in the integration of human neurons along with supporting brain cells into the brain tissue of rats to form hybridized working circuits. The implanted human organoids survived, thrived and grew. Individual neurons from the human organoids integrated into young rats’ brains were at least six times as big as those — generated the same way, at the same time — that remained in a dish. The transplanted neurons also exhibited much more sophisticated branching patterns. Pasca and his colleagues observed marked differences in the electrical activity of, on one hand, human neurons generated from a Timothy syndrome patient, cultured as organoids and transplanted into one side of a rat’s brain, and, on the other hand, those generated from a healthy individual and transplanted, as an organoid, into the corresponding spot on the other side of the same rat’s brain. The Timothy syndrome neurons were also much smaller and were deficient in sprouting branching, brush-like extensions called dendrites, which act as antennae for input from nearby neurons.
“We’ve learned a lot about Timothy syndrome by studying organoids and assembloids kept in a dish,” Pasca said. “But only with transplantation were we able to convincingly see these neuronal-activity-related differences.”
That same year, the FDA Modernization Act 2.0 was signed into law, exempting certain categories of new drug-development protocols from previously mandated animal testing. The act was predicated on the understanding that recent advancements in science offer increasingly viable alternatives to animal testing, so the findings based on the organoid- and assembloid-culture technologies may be adequate to justify clinical trials in some neurodevelopmental conditions.
Most recently, in a Nature paper published April 24, Pasca and his colleagues demonstrated, in principle, the ability of antisense oligonucleotides (ASOs) to correct the fundamental defects that lead to Timothy syndrome by nudging calcium-channel production toward another form of the gene that does not carry the disease-causing mutation. Using ASOs to guide production of the functional rather than defective form of this channel reversed the defect’s detrimental downstream effects: Interneuronal migration proceeded similarly to that procedure in healthy brains, and the altered electrical properties of the calcium channel reverted to normalcy. This therapeutic correction was demonstrated in a lab dish — and, critically, in rat-transplantation experiments, suggesting that this therapeutic approach can work in a living organism.
Pasca is now actively searching the globe for carriers of the genetic defect, in preparation for the pursuit of a clinical trial at Stanford Medicine to test the safety and therapeutic potential of ASOs in mitigating the pathological features of Timothy syndrome.
“We are also actively engaged in conversations with other scientists, clinicians in the field and ethicists about the best way to move forward and safely bring this therapeutic approach into the clinic,” he said.
Pasca added that the calcium channel that is mutated in Timothy syndrome is, in fact, “the hub” of several neuropsychiatric diseases including schizophrenia and bipolar disorder. So it may be that the lessons learned — and the therapies derived — from his 15-year focus on a rare disease may have broad application in a number of widespread and troubling psychiatric conditions.
‘Amazing’ teenager
Today, in defiance of his doctors’ warning that he might not live past age 2, Holden Hulet is 15 years old and doing well.
“I think a lot of times, autism is perceived as ‘They’re not neurotypical and they’re not capable of certain things.’ But he is brilliant,” JJ said. “He’s amazing with techie stuff or Legos. He’s funny and super honest and very self-aware.”
Kelby often takes Holden to visit the farm where he grew up. Holden loves to ride the farm equipment and enjoys hanging out with the animals, especially the farm dogs and calves. Like a lot of kids, he keeps an eye out for good rocks, Kelby said with a chuckle.
“He’s always either throwing them or collecting them,” JJ said. “That’s something I really like about him: He’s always got a pocket full of something.”
Although navigating a rare disease is one of the most challenging things they have faced, the Hulets see light in their situation, and would offer encouragement to any family facing a new Timothy syndrome diagnosis.
“There is hope,” JJ said. “There are people out there who care, people out there who fight for you who don’t even know you. I think that’s what is so important about research — that you’re fighting a battle for people you don’t even know.”