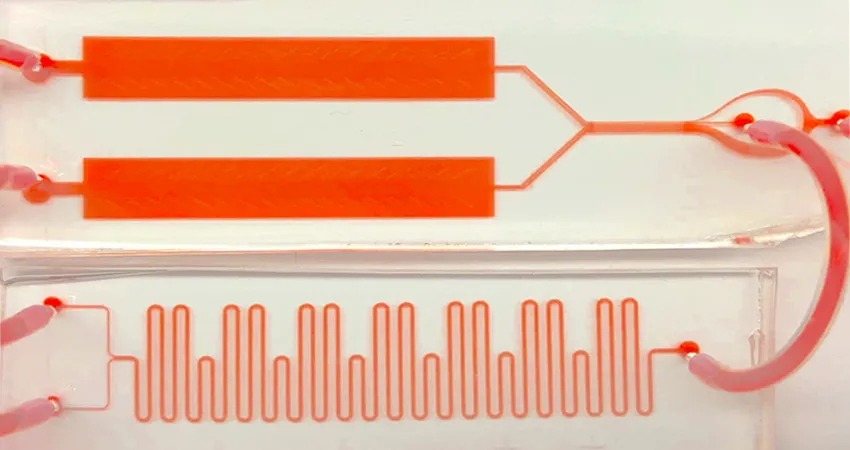
Image courtesy Caitlin Maikawa: The RT-ELISA prototype consists of three modules: in the first (bottom), blood from the subject is mixed with a solution containing beads of target protein-detecting probes and fluorescent detection antibodies. The second module (top-right) eliminates excess blood cells. And the third module (top-left), transfers the fluorescently labeled beads to a detection window for measurement by a high-speed camera.
Stanford News - December 21st, 2020 - by Andrew Myers
For even the most routine of medical checkups, a blood test is often the first order of business. But, for all its diagnostic power, this common test provides only a snapshot of the blood during a single moment in time.
“A blood test is great, but it can’t tell you, for example, whether insulin or glucose levels are increasing or decreasing in a patient,” said Tom Soh, a professor of electrical engineering and of radiology at Stanford. “Knowing the direction of change is important.”
Now, Soh, in collaboration with Eric Appel, an assistant professor of materials science and engineering, and colleagues have developed a technology that can provide this crucial piece of missing information. Their device, which they’ve dubbed the “Real-time ELISA,” is able to perform many blood tests very quickly and then stitch the individual results together to enable continuous, real-time monitoring of a patient’s blood chemistry. Instead of a snapshot, the researchers end up with something more like a movie.
In a new study, published in the journal Nature Biomedical Engineering, the researchers used the device to simultaneously detect insulin and glucose levels in living diabetic laboratory rats. But the researchers say their tool is capable of so much more because it can be easily modified to monitor virtually any protein or disease biomarker of interest.
Technologically, the system relies upon an existing technology called Enzyme-linked Immunosorbent Assay – ELISA (“ee-LYZ-ah”) for short. ELISA has been the “gold standard” of biomolecular detection since the early 1970s and can identify virtually any peptide, protein, antibody or hormone in the blood. An ELISA assay is good at identifying allergies, for instance. It is also used to spot viruses like HIV, West Nile and the SARS-CoV-2 coronavirus that causes COVID-19.
“We do ELISA continuously,” Soh said.
The Real-time ELISA is essentially an entire lab within a chip with tiny pipes and valves no wider than a human hair. An intravenous needle directs blood from the patient into the device’s tiny circuits where ELISA is performed over and over.
Soh likens the process to making a protein sandwich in which two molecules, or antibodies, are attached to the protein of interest. One antibody can be custom-tailored to seek out and latch onto the specific biomarker. Once it is attached, the second antibody is activated. This antibody fluoresces, or glows, which is monitored by a high-speed camera. Based on how brightly the blood sample glows, scientists can determine not only whether the target protein is present but also its concentration. The more target molecule exists in the blood, the brighter the sample will be.
Real-time, continuous blood monitors have been developed for a few blood markers like glucose, lactate and oxygen, but extending the technology beyond those few examples has proven “exceedingly difficult,” Soh said. This is why the Real-time ELISA’s adaptability to myriad proteins is especially promising.
“Don’t think of this as just an insulin sensor. Think of this as a way of doing ELISA in a completely new and different way,” Soh said.
The Real-time ELISA might also prove a boon to biomedical research, providing instantaneous and detailed feedback on the effectiveness of drugs and other therapies, but Soh thinks the device will be most useful in intensive care units and emergency rooms, where time and accuracy are of the essence.
As one example, Soh points to the “cytokine storm” triggered by the disease COVID-19. As the illness sets in, the patient’s immune system kicks into high defensive alert and produces loads of disease-fighting signaling proteins, called cytokines. In some instances, the body fails to reduce the threat level even after the disease is brought under control, instead producing more and more cytokines, which can lead to sepsis and internal organ failure.
Soh envisions a future version of Real-time ELISA telling doctors whether cytokine levels in a patient are decreasing in response to treatment. Soh and his team are already at work modifying the device to measure a key cytokine, known as IL-6. Currently, IL-6 tests must be sent out to a lab for processing and it takes three days to get results back.
“In sepsis, time is key – every hour that goes by, your probability of dying increases by 8 percent,” Soh said. “Patients don’t have three days for a single test. That could have life-saving implications.”
Other Stanford co-authors on the study, titled “A fluorescence sandwich immunoassay for the real-time continuous detection of glucose and insulin in live animals,” include postdoctoral scholar Mahla Poudineh and doctoral candidate Caitlin Maikawa (co-lead authors), and professors Jelena Vuckovic and Seung Kim.
The research was supported by the Chan-Zuckerberg Biohub, the Stanford Diabetes Research Center Pilot Grant and the Transdisciplinary Initiative Program (TIP) of the Stanford Maternal & Child Health Research Institute.





