Photo of first author Eric Zhao, Stanford Bio-X Fellow and graduate student in the Melosh lab.
June 7, 2023 - Science Advances
Stanford Bio-X affiliated faculty members Nick Melosh and John Huguenard, with first author Stanford Bio-X Fellow Eric Zhao and co-author Travel Award recipient Pingyu Wang, have developed an electrode connector based on an ultra-conformable thin-film electrode array that self-assembles onto silicon microelectrode arrays, enabling multithousand channel counts at a millimeter scale. This work could greatly increase the scalability of existing electrophysiological devices, potentially allowing them to capture cortex-wide activity in the brain such as thoughts, actions, and perception.
Perception, thoughts, and actions involve the coordinated activity of large populations of neurons in multiple regions of the brain. Nonpenetrative, subdural ECoG grids laid on top of the brain surface are the gold standard for recording population-level activity, measured from the local field potential (LFP). Neurophysiological recordings with ECoG grids have been successfully used for speech synthesis, reproduction of arm movements, and spatial localization of ictal onset zones. They have also been used to characterize cortical traveling waves, which have been shown to modulate perceptual sensitivity. Hence, ECoG grids are a favorable modality for brain-computer interface applications, localization of epileptic foci for clinical epilepsy diagnosis and targeted tissue resection, and as a basic science tool.
Ultraconformable thin-film flexible devices that can conform to the curvilinear surface of the brain are a promising technology to capture cortex-wide spatiotemporal dynamics. Microfabrication and advanced lithography methods have enabled the creation of thin-film arrays with hundreds to thousands of recording sites. However, the key bottleneck lies not in the fabrication of these devices but in the connectorization between each electrode and the external circuitry. Because of the bulkiness of existing connectorization methods such as wire bonding, anisotropic conductive film, and ultrasonic-on-bump bonding, current implementations of multithousand channel count, passive thin-film neural interfaces are highly complex, bulky, and unscalable.
Here, we sought to combine the scalability and exceptional performance of CMOS-MEAs with the ultraconformable form factor of flexible devices. We achieve this by developing a scalable, high-density connectorization strategy, which can form thousands of interconnections between the electrode pads on the flexible device and the pixels on the CMOS-MEA at a high density. The flexible device extends out from the CMOS-MEA through long leads, converging at the distal end as an array that interfaces with the brain.
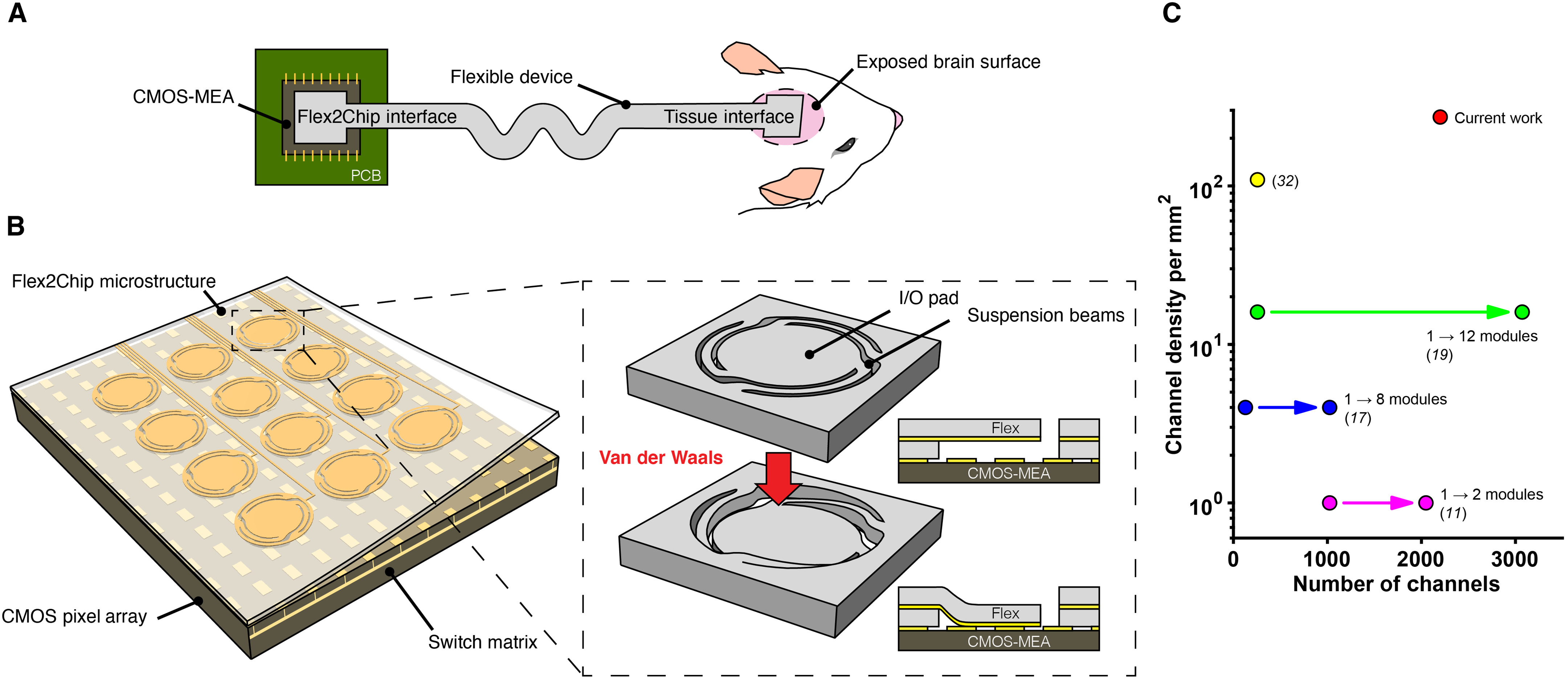
Flex2Chip design: (A) Schematic of the flexible device. At the chip interface, Flex2Chip microstructures enable multithousand ohmic interconnections to a CMOS-MEA. At the tissue interface, the device terminates as an electrode array for high density recording. (B) Flex2Chip interface consists of an array of deformable Flex2Chip microstructures, integrated with the underlying CMOS-MEA. The Flex2Chip microstructures consist of three suspended beams and an I/O pad. Capillary forces deform the I/O pad to contact the CMOS pixels, upon which van der Waals forces become significant to establish structural and electrical contact. (C) Current approaches to multithousand channel counts rely on the modularization of multiple ASICs and circuit boards. The Flex2Chip structures facilitate 2200 individual ohmic connections to a single ASIC with an area of 3.5 mm by 2.1 mm, yielding a channel density of 272 channels/mm2.