Photo by Sebastian Kaulitzki, Shutterstock.
Stanford Medicine News Center - December 18th, 2015 - by Bruce Goldman
Mice that had strokes rebounded significantly faster if they received low doses of a popular sleeping aid, according to researchers at the Stanford University School of Medicine.
Zolpidem, better known by the trade name Ambien, has long been approved by the U.S. Food and Drug Administration for treating insomnia. But it has never before been definitively shown to enhance recovery from stroke, said Gary Steinberg, MD, PhD, professor and chair of neurosurgery. Steinberg shares senior authorship of the study, published online Dec. 18 in Brain, with senior research scientist Tonya Bliss, PhD.
Steinberg, the Bernard and Ronni Lacroute-William Randolph Hearst Professor in Neurosurgery and the Neurosciences, cautioned that the study’s results need to be independently replicated in other laboratories before clinical trials of the drug’s capacity as a stroke-recovery agent can begin.
Graphic: Ashley Boelens
Every year, Americans incur about 800,000 strokes, the nation’s largest single cause of neurologic disability, exacting an annual tab of about $74 billion in medical costs and lost productivity.
A stroke’s initial damage, which arises when the blood supply to part of the brain is blocked, occurs within the first several hours. Drugs and mechanical devices for clearing the blockage are available, but to be effective they must be initiated within several hours of the stroke’s onset. As a result, fewer than 10 percent of stroke patients benefit from them.
After a few days during which tissue death continues to spread to adjacent brain regions due to repercussions from the initial damage, the brain begins slowly rewiring itself and substituting new neural connections for those destroyed by the stroke. Within three to six months, at least 90 percent of all the recovery a stroke patient is likely to experience takes place. No pharmaceutical therapy has been shown to improve recovery after the stroke's damage has been done. In fact, no effective treatments during the recovery phase exist, other than physical therapy, which has been shown to be only marginally successful.
Nerve-cell signaling bolstered
Steinberg and Bliss attributed zolpidem’s effectiveness to its enhancement of a type of nerve-cell signaling activity whose role in recovery unexpectedly appears beneficial. In the study, this signaling was bolstered even though the drug was given at doses well below those at which it exerts its hallmark sedative effect.
Nerve cells signal to one another by means of substances called neurotransmitters. When neurotransmitters are secreted by the nerve cell sending the signal, they dock in receptors situated on abutting nerve cells’ surfaces. Most of this signaling takes place at specialized junctions called synapses, which feature high concentrations of neurotransmitters from the upstream cell that activate receptors on the downstream cell.
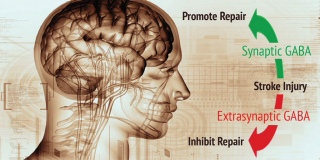
Neurotransmitters can be excitatory, triggering propagation of an impulse in the receiving nerve cell. Or they can be inhibitory, temporarily preventing the receiving nerve cell from propagating any impulses. The roughly one-fifth of all nerve cells in the brain that are inhibitory mainly do their job by secreting a neurotransmitter called GABA.
While the bulk of GABA signaling takes place at synapses, scientists have learned that nerve cells can also feature GABA receptors elsewhere on their outer surfaces. These are called extrasynaptic receptors. In 2010, other researchers reported that extrasynaptic GABA signaling impeded stroke recovery in an animal model. But until the Stanford study, nobody had looked into the impact on stroke recovery of the far more common synaptic GABA signaling.
To do that, Steinberg, Bliss and their associates conducted a series of anatomical, physiological and behavioral experiments. Their efforts were assisted by the fact that there are small, structural differences between synaptic and extrasynaptic GABA receptors, so they can be distinguished by various techniques.
Using a high-resolution visualization method, the Stanford scientists examined a region of the mouse brain near the area that had been destroyed by stroke and is known to rewire afterward. They saw a transient increase in the number of GABA synapses. This increase peaked at about a week after the stroke and subsided to baseline levels by one month after the stroke. The rise and fall of synapse-associated GABA receptors was restricted to a particular layer of the cerebral cortex that sends output to the spinal cord and to other brain areas.
Intrigued by this anatomical finding, the scientists looped in their colleague John Huguenard, PhD, professor of neurology and neurological sciences and co-author of the study. Electrophysiological experiments in Huguenard’s lab confirmed that the transitory increase in GABA synapse numbers in the brain area under scrutiny was matched by an increase, followed by a decline to baseline levels, in synaptic GABA signaling, confirming that the synapses were indeed functional.
Sub-sedative doses
To determine whether the transient increase in post-stroke synaptic GABA signaling was beneficial — and, if so, whether it could be enhanced — the investigators turned to zolpidem, which works by enhancing synaptic GABA signaling. They induced either of two different types of strokes in mice — one type severely damages sensory ability; the other deeply impairs movement — then put the mice on a regimen of either zolpidem or a control solution that did not contain the drug.
The scientists administered the drug in sub-sedative doses. They wanted to see how the mice would perform on tests of sensory ability and motor coordination, so the mice needed to be fully awake. Zolpidem is known to have a much higher affinity for synapse-associated GABA receptors than for their extrasynaptic counterparts. So, low doses were likely to enhance synaptic GABA signaling without having much of an effect on extrasynaptic signaling.
The team delayed zolpidem administration until three days after the stroke in order to ensure that any benefit they observed was resulting from an effect on brain recovery, rather than from the drug preventing initial tissue damage from the stroke.
The researchers subjected these mice to two kinds of tests. One measured the speed with which they removed a patch of adhesive tape from one of their paws (mice ordinarily are quick to do so). The other test gauged their ability to traverse a horizontal rotating beam.
In almost every case, zolpidem-treated mice recovered at a faster rate than control mice did. It took about a month, for example, for mice not given zolpidem to fully recover their stroke-impaired ability to notice the tape stuck to their paw. Mice given zolpidem recovered that ability within a few days of treatment.
While zolpidem dramatically improved mice’s rate of recovery from stroke, its ability to increase the extent of their recovery couldn’t be determined because, unlike humans, mice naturally regain most of their pre-stroke function eventually. So the Stanford researchers intend to test the drug in other animal models, as well as to experiment with different dose sizes and timing, before proceeding to clinical trials.
“Before this study, the thinking in the field was that GABA signaling after a stroke was detrimental,” said Steinberg. “But now we know that if it’s the right kind of GABA signaling, it’s beneficial. And we’ve identified an FDA-approved drug that decisively promotes the beneficial signaling.”
Lead authorship of the study is shared by Takeshi Hiu, MD, PhD, a former postdoctoral scholar in the Steinberg laboratory, and Zoya Farzampour, PhD, a former graduate student in the Huguenard laboratory.
Other Stanford co-authors are former postdoctoral scholar Jeanne Paz, PhD, now at the University of California-San Francisco; former research assistants Eric Wang and Corrine Badgely; Andrew Olson, director of microscopy for the Department of Neurology; basic life science research associates Gordon Wang, PhD, and Xibin Liang; former visiting scholars Robin Lemmens, MD, PhD, and Yasuhiro Nishiyama, MD; former life science research assistant Kevin Tran, MD; Scott Hamilton, PhD, a consulting associate professor at the Stanford Stroke Center; senior research scientists Kristina Micheva, PhD, and Nancy O’Rourke, PhD; and former professor of molecular and cellular physiology Stephen Smith, PhD.
The study was supported by the National Institutes of Health (grants R01NS058784, K99NS078118, R01NS006477 and R01NS034774), Bernard and Ronni Lacroute, Russell and Elizabeth Siegelman and the National Science Foundation.
Stanford’s Department of Neurosurgery also supported the work.



